Progenabiome and Colleagues Push Innovation in Combating COVID 19
MALIBU, Calif., May 4, 2020 /PRNewswire/ — Progenabiome is sharing gut microbiome research findings by next generation shotgun sequencing utilizing Kraken metagenomic analysis at the Digestive Disease Week (DDW) 2020 virtual meeting May 2-5. The California-based research lab was scheduled to present at DDW, the world’s largest gathering of digestive disease health professionals with 14,000+ attending each year, however the meeting was cancelled due to the coronavirus pandemic. Data will now be shared on the DDW ePosters and ePapers site, available to access at no charge.
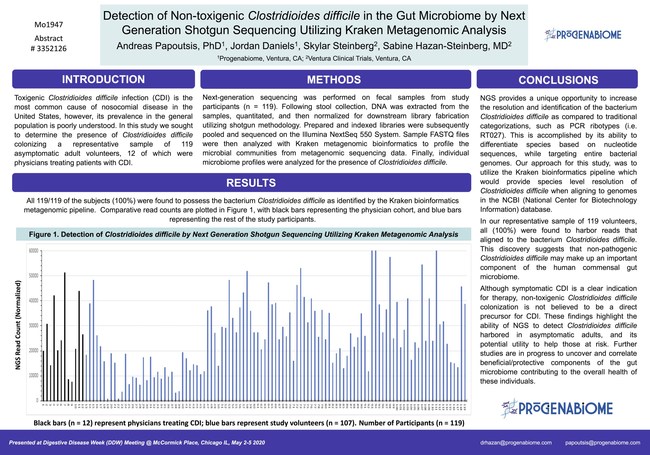
“Finding non-toxigenic Clostridium difficile (C.diff) in the gut flora of all 119 subjects tested by genetic sequencing raises the question whether C.diff is an innocent bystander or villain and if antibiotics should be used to kill something that is part of our microbial fingerprint,” says Progenabiome CEO Sabine Hazan, MD, “this breakthrough in research challenges us to look beyond the traditional protocols to treat bacteria and viruses automatically with drugs. It also forces us to understand the balance of the microbiome that allows for immunity to occur.”
Hazan has been a solo practice gastroenterologist and clinical trials investigator for 25+ years. She has participated in over 150 clinical trials for the pharmaceutical and nutrition industries and launched Progenabiome in 2018 to investigate the role of the gut microbiome in various diseases and conditions.
Strategically placed as a genetic sequencing lab, site, CRO, and now sponsor, Progenabiome has 39 ongoing clinical trials, including three COVID-19 studies through which the lab is validating testing, prophylaxis, and at-home treatment protocols for the novel coronavirus.
“A one-pill-solution is rarely the answer for everyone,” argues Hazan, who believes medicine is as much an art as it is a science. She argues that physicians and patients need multiple options given the complexity of the interactions taking place inside us.
Progenabiome is also leading COVID-19 research with several innovative clinical trials in the pipeline. Having worked with Gilead as an investigator for their Harvoni treatment, she sees the approval of Remdesivir for COVID-19 as a step in the right direction, however she maintains that intravenously administered medication “is not a panacea.” She argues, “this virus requires a solution for all” so further research of alternate options remains necessary. Like Hazan, many physicians maintain that more studies are needed for effective and accessible COVID-19 solutions, including further investigation of hydroxychloroquine (HCQ) and azithromycin (AZM) which have shown promise in COVID studies globally.
Dr. Alan Miller, president and CEO of Alta Pharmaceutical Research Center reiterates the need for more clinical research, saying, “We currently have only one FDA-approved medication for COVID-19 and that isn’t enough.” Dr. Miller has participated in 110 FDA-approved clinical trials and has helped bring several drugs to market including: Crestor, Zetia, Januvia, Uloric, Cialis, Repatha, Victoza, Trulicity, various insulins, and others.
However, concern over the extension of the QT interval has been highlighted as a potential cardiac side effect of HCQ, albeit azithromycin has a similar effect. Physician experts maintain that long-established HCQ, which has been approved for medical use in the U.S. since 1955, is very safe for the vast majority of patients, subject of course to utilizing the drugs within agreed parameters.
Dr. Ami Ben-Artzi has treated thousands of patients with HCQ and says the rheumatology community considers HCQ safe with minimal side effects and concerns only in long-term use. He has 15 years experience at multiple institutions including NYU, UCLA, and presently, Cedars-Sinai Medical Center.
Dr. John Goldman, former Emory University professor and author of 50 articles, 35 chapters and 89 abstracts, has over 50 years experience treating patients with HCQ. He has prescribed tens of thousands of doses and has not evidenced adverse effects for G6PD or EKG. He describes HCQ as “a very safe drug”
Dr. Alon Steinberg, Progenabiome’s Chief Medical Officer, argues “More data is needed to evaluate the risks and benefits of HCQ and AZM for COVID.” As a leading cardiologist, he is particularly interested in visualizing EKG strips of COVID patients who receive treatment at home.
This seems to be backed up by the recent publication by Ehud Chorin and 12 other cardiologists on the QT interval in 84 patients treated with HCQ and azithromycin, which demonstrated that “there were no torsades de pointes events recorded, including in patients with severely prolonged QTc… Four patients died from multi-organ failure, without evidence of arrhythmia.”
Dr. Thomas Borody, world-renowned leader in the clinical microbiota says, “I see these as important trials to enable us to determine whether we can stem the tide of infection, protect our frontline staff and start using the drugs we have to protect our global community. Time is of the essence.” Borody is the founder of the Centre for Digestive Diseases, reviewer for several leading medical journals, and holds over 160 patents in areas including treatment of H.pylori, Crohn’s, IBS, FMT and more.
Dr. Hazan and Dr. Borody are collaborating on several innovative studies including COVID-19 therapy protocols.
The two microbiome experts were previously set to present groundbreaking microbiome data at the Malibu Microbiome Meeting (MMM), originally scheduled for March 28-29. Now, they hope to share their research and COVID findings at the rescheduled MMM event on August 22-23 along with fellow leading physicians and academicians from around the world. Other MMM speakers include Drs. Paul Feuerstadt (Yale), Colleen Kelly (Brown), Sahil Khanna (Mayo Clinic), Jessica Allegretti (Harvard), Neil Stollman (UCSF), Scott Jackson (NIST), Howard Young (NIH), and more.
For more, visit
https://progenabiome.com
https://clinicaltrials.gov
https://malibumicrobiomemeeting.com/
the microbiome
the microbiome diet
the human microbiome
SOURCE Progenabiome